Atoms
The attraction of electrons to protons was a magic moment in the history of the Universe. The result was the creation of atoms, a step towards the self-organization of matter which would eventually produce the Earth and people.
Hydrogen Atom
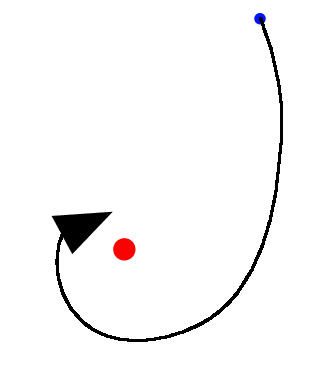
Much of the rest of this story will be concerned with the properties of atoms. So let us begin by watching as a single electron, with its negative electric charge, was attracted towards a single positively charged proton.
An electron was attracted towards a proton
Both the electron and the proton were moving, but because the electron was much lighter than the proton, it moved much quicker and its path was bent more easily.
If the electron was traveling too fast it would escape from the proton, but if it was slow enough it would get trapped and go into orbit, a little like the Moon around the Earth.

The electron began to orbit the proton
But electrons are not like the Moon. They are much smaller and subject to the laws of quantum mechanics. This made the electron spread out into a cloud around the proton.

The electron formed a cloud around the proton
(Note that in our diagrams the size of the nucleus is exaggerated for clarity.)
The electron formed a cloud of probability. The density of the cloud determined how likely it was to find the electron at that point in space.
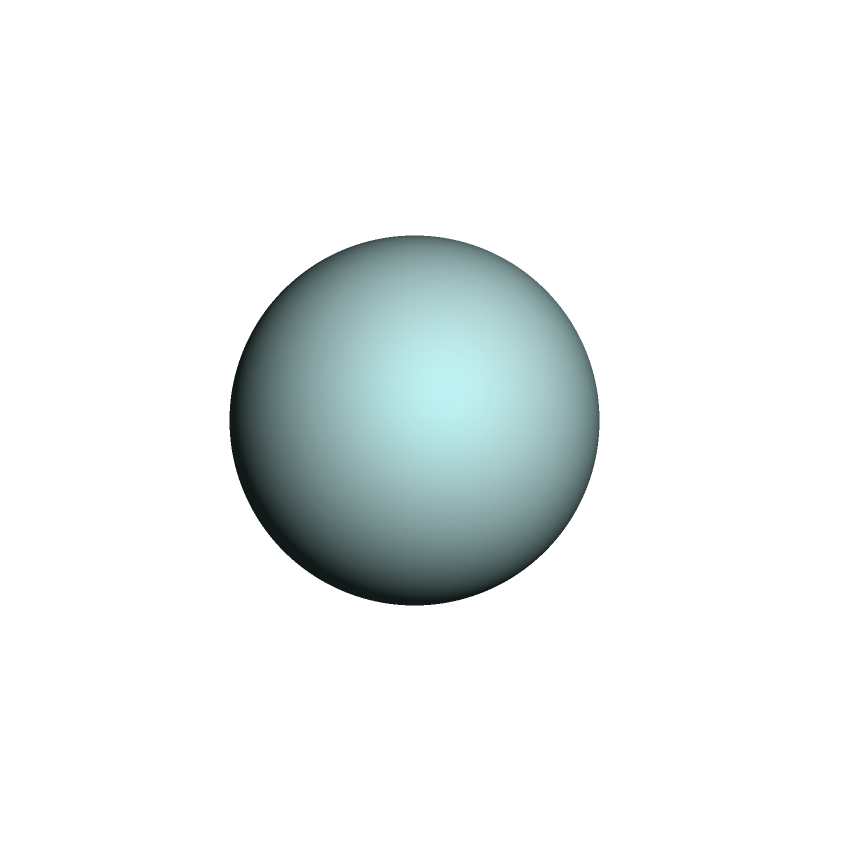
The cloud formed a shell completely surrounding the proton like a hollow ball. From the outside the ball looks like this:

The electron shell completely surrounded the proton like a hollow ball. Image produced by Discovery Studio
Now the proton and the electron were held together and traveled as a single unit. This is an example of an atom. There are about 100 different types of atom known on Earth today, all with different names and different numbers of protons in the centre and electrons on their outside.
This was the simplest possible atom, with just one proton and a single electron. It is called a hydrogen atom. The name comes from the Greek words "hydros genos" which means "water forming", so-called because this type of atom is found as a constituent of water.
Helium Atom
As well as protons, the young Universe also contained helium nuclei. These contained two neutrons and two protons, both of which attracted electrons.
The first electron formed a shell around the helium nucleus in the same way as the hydrogen atom, except that it was smaller because it was attracted by both protons and so pulled in with a stronger force.

Electron formed a smaller shell around helium nucleus
(Remember that in our diagrams the size of the nucleus is exaggerated for clarity.)
The charge on the two protons showed through this electron shell and they were able to attract another electron.
Helium attracts second electron and fills its shell
Hard shell of atom
These two electrons now fill the space around the nucleus, so that no other electrons can enter this space. (The reason why this happens is explained by the theory of quantum mechanics).
The helium atom now behaves as if it had a hard outer shell. It will not interact with other atoms. Atoms like this are called noble gases. An atom with two electrons making a hard outer shell like this is called a helium atom. It got this name because it was first detected by examining the spectrum of light from the Sun, whose Greek name is Helios.
Atoms are extraordinary objects. Around the outside of the atom, the electron or electrons form a large thin shell. Inside the atom is almost empty space, except for the tiny heavy nucleus at the centre.
An atom is like a table-tennis ball with thin outer shell and containing a tiny speck of dust floating at its centre. We will see later that some atoms are able to stick together in different ways to make a rich variety of objects called molecules.
Size of Atoms on Soccearth
How big is an atom? You would think that heavy atoms, with many electrons, would be much larger. Surprisingly, all atoms are about the same size! Can you think why this might be? Click here to find out why.
So how large is an atom?
If a soccer ball were inflated to the size of the Earth (which I call Soccearth), atoms are between 10 and 20 millimeters across.
In this table we show the diameter (distance across) of the most important atoms in this story. These sizes are based on the table given in the Atom Sizes page of this website. The diameters are shown in millimeters.

The sizes of atoms on Soccearth
Note that real atoms are not colored. The colors shown here are the ones often used in models of molecules.
